编者按:2025年美国临床肿瘤学会泌尿生殖系统肿瘤研讨会(ASCO GU)已于2月13日至15日在加利福尼亚州旧金山召开,吸引了全球泌尿系统领域的顶尖专家和学者参与。在此次会议上,犹他大学亨茨曼癌症研究所(HCI) Neeraj Agarwal教授报告了TALAPRO-2研究的最终总生存期数据,引发全球范围内的高度关注。该研究不仅填补了PARP抑制剂联合NHT一线治疗全人群的空白,更强化了精准医疗在前列腺癌治疗中的重要价值。《肿瘤瞭望-泌尿时讯》特邀Neeraj Agarwal教授,为我们解读最新结果和临床意义,以及对mCRPC一线治疗策略变革的影响。
01
《肿瘤瞭望-泌尿时讯》:作为前列腺癌的终末阶段,您认为mCRPC治疗的重点和难点是什么?
Neeraj Agarwal教授:转移性去势抵抗性前列腺癌(mCRPC)是前列腺癌的终末阶段,异质性高且预后较差,一线接受新型内分泌治疗(NHT)的中位OS通常不足3年,且50%的患者无法进入到二线及后线治疗,这一现状令人担忧。我认为当前的首要任务是改善患者的生存预后,并尽可能让他们接受更多种类的治疗方案,以延长患者的生存期。
然而,无论是我们在临床试验中取得成果,还是在顶级期刊上发表论文,只要这些研究成果未能真正转化为临床实践,那么这些努力将失去其应有的价值。在转移性激素敏感性前列腺癌(mHSPC)中已经出现了类似的问题,而在mCRPC患者中,这一问题显得更为紧迫。如若不能及时启动治疗,患者可能在短短两三个月内病情迅速恶化,甚至死亡,情况十分紧急。这也是联合疗法在前列腺癌及其他恶性肿瘤中发挥重要作用的原因之一。通过同时提供两种或多种治疗手段,我们可以显著延缓疾病进展,改善晚期前列腺癌患者的预后,其具体疗效则取决于所选用的治疗方案。
Dr. Neeraj Agarwal: Metastatic castration-resistant prostate cancer is the symptomatic state of prostate cancer and represents a lethal phase of the disease. The median overall survival of patients with metastatic CRPC is less than three years in the United States.
Half of our patients with metastatic CRPC across the country do not receive more than one line of systemic therapy, and these data are quite worrisome. I believe the priority is to improve survival and allow our patients to receive as many lines of therapy as possible, which will ultimately lead to an improvement in survival.
There is no point in conducting a trial, publishing the data in a top journal, and then not seeing those findings being utilized in patient care. We see the same theme in metastatic hormone-sensitive prostate cancer, but in metastatic CRPC, as I said, this problem is even more pressing. If we do not start a drug in a timely fashion, we can lose our patients within two or three months. That is how urgent this issue is.
This is one reason why combination therapies work in prostate cancer, like in many other cancers—because we essentially provide two lines of therapy together. Patients do significantly better because they receive dual therapy, leading to a substantial delay in disease progression, depending on the regimen.
02
《肿瘤瞭望-泌尿时讯》:目前已有几种多聚腺苷二磷酸核糖聚合酶(PARP)抑制剂获批用于mCRPC治疗。您认为这对于推动该领域精准治疗的发展具有哪些意义?
Neeraj Agarwal教授:PARP抑制剂在推动mCRPC精准医学方面发挥了重要作用。多项临床试验表明,PARP抑制剂与雄激素受体(AR)抑制剂之间存在协同作用,联合使用可显著改善mHSPC的生存率,延缓疾病进展,mCRPC亦是如此。
此次ASCO GU年会上,我报告了TALAPRO-2研究的最终OS数据。TALAPRO-2是一项国际多中心、随机双盲、安慰剂对照的Ⅲ期临床试验,旨在评估PARP抑制剂他拉唑帕利联合雄激素受体抑制剂恩扎卢胺对比恩扎卢胺联合安慰剂一线治疗mCRPC的疗效。结果显示,他拉唑帕利联合恩扎卢胺一线治疗mCRPC全人群的OS达到45.8个月。
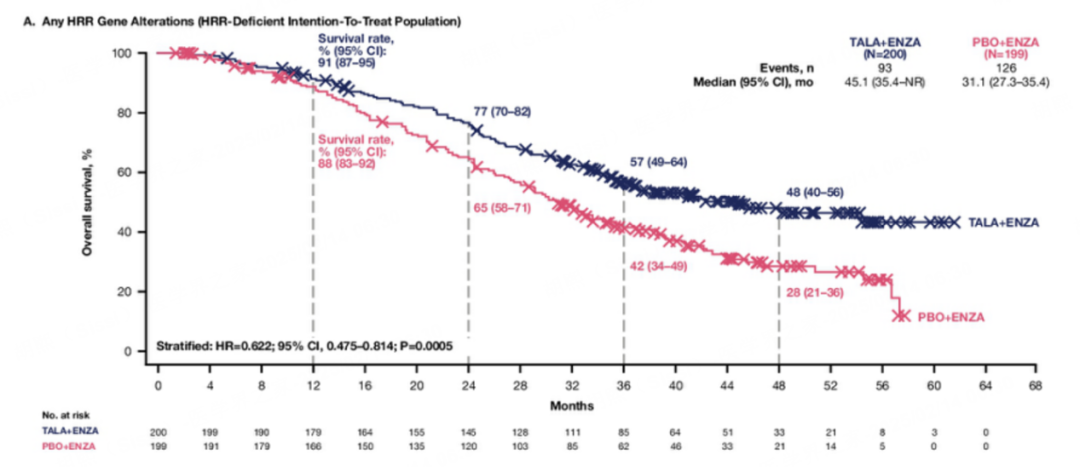
值得注意的是,该研究还特别探索了同源重组修复突变(HRRm)人群的获益情况,并取得了阳性结果,为精准治疗提供了重要依据。具体为:在HRRm人群中,与安慰剂联合恩扎卢胺相比,他拉唑帕利联合恩扎卢胺的中位OS延长了约14个月(45.1个月vs 31.1个月,HR=0.622,P=0.0005),死亡风险降低了约40%,他拉唑帕利联合恩扎卢胺组与安慰剂联合恩扎卢胺组的中位放射学无进展生存期(rPFS)分别为30.7个月vs 12.3个月(HR=0.468,P<0.0001)。
在non-BRCA HRR突变mCRPC人群中,他拉唑帕利联合恩扎卢胺组与安慰剂联合恩扎卢胺组的中位OS分别为42.4个月vs 32.6个月(HR=0.727,P=0.0665)。在BRCA突变mCRPC人群中,他拉唑帕利联合恩扎卢胺组与安慰剂联合恩扎卢胺的中位OS分别为NR vs 28.5个月(HR=0.497,P=0.0017)。在HRRm人群的所有亚组中,他拉唑帕利联合恩扎卢胺真正地延长了患者的生存期。(更多内容详见:TALAPRO-2研究OS突破:他拉唑帕利联合恩扎卢胺一线治疗mCRPC,开启生存新纪元!)该研究历时四年,暂未发现新的安全性信号。尽管确实存在一定的不良反应,但总体可控。
中位OS达到45.1个月,生存期延长至近4年,对比前文提到的“一线接受NHT的中位OS通常不足3年”,可谓是取得了重大进步。随着多款PARP抑制剂在mCRPC领域取得积极成果,PARP抑制剂联合NHT已成为各项权威指南推荐的mCRPC一线标准治疗方案(他拉唑帕利也成为目前中国首个且唯一获批用于HRR突变mCRPC治疗的PARP抑制剂,为HRR突变患者提供了一种创新且有效的治疗方案)。鉴于该联合治疗方案在mCRPC中展现出的显著生存获益,且未对患者生活质量产生负面影响,该方案值得在临床实践中与每位患者进行深入探讨。当前的主要挑战仍然是如何确保患者能够及时接受这些创新疗法,以最大化其生存获益。
Dr. Neeraj Agarwal: PARP inhibitors play a significant role in advancing precision medicine for mCRPC. For instance, multiple trials have shown improvements in disease progression time when PARP inhibitors are combined with AR pathway inhibitors.
I presented data from the TALA-PRO2 trial, which demonstrated that combining talazoparib, a PARP inhibitor, with enzalutamide significantly improved overall survival in an all-comer or unselected patient population.
The rationale behind this is twofold. First, patients with homologous recombination repair (HRR) mutations experienced a greater magnitude of benefit. Specifically, there was about a 14-month improvement in overall survival, with a hazard ratio of 0.6—meaning approximately a 40% reduction in the risk of death for patients who had HRR gene mutations in their prostate tumors.
Even among patients without HRR gene alterations, there was a benefit. While these are smaller subgroups and the trial was not powered to dissect individual subgroups, there was still a strong trend for benefit with a hazard ratio of 0.78. Clearly, there is benefit across all patient groups, but the magnitude of benefit is higher in HRR mutation-positive patients. Among HRR-negative patients, the median overall survival benefit was nine months. These are not trivial benefits—they span several months, significantly impacting patient outcomes.
In the metastatic hormone-sensitive setting, when you combine PARP with AR pathway inhibitors, you see a strong survival benefit. The same principle applies to metastatic CRPC.
The TALA-PRO2 trial demonstrated that adding talazoparib to enzalutamide provides a clear survival benefit in all-comer patients, with a stronger benefit in those with HRR mutations. The safety data, after four years of follow-up, showed no new safety signals. While there were some side effects, as oncologists, we know how to manage them—or if not, we can learn how to manage them.
Given the significant overall survival benefit and the fact that quality of life was not compromised, these agents are worth discussing with every patient in the clinic. The key challenge remains ensuring that patients receive these therapies in a timely manner to maximize survival benefits.
03
《肿瘤瞭望-泌尿时讯》:目前是否有相关标志物,可以预测他拉唑帕利联合恩扎卢胺治疗mCRPC的疗效及预后?
Neeraj Agarwal教授:基因测序和下一代测序(NGS)是推动前列腺癌治疗领域精准医学发展的重要工具。关于是否应为所有患者进行NGS检测,业界仍存在诸多讨论。答案是肯定的:每一位转移性前列腺癌患者都应接受NGS检测。这一观点得到了ASCO指南以及几乎所有主流医学指南的支持。
基因测序和NGS检测的重要性体现在多个方面。对于携带BRCA1/2和HRR基因突变的患者,NGS检测能够明确其突变状态,从而为靶向治疗(如PARP抑制剂)提供依据。TALAPRO-2研究中,联合方案的显著生存获益有力地证明了这一点。其次,基因检测不仅对患者本身具有重要意义,对家属同样至关重要。许多患者的基因突变可能影响家族成员的患癌风险,因此明确这些信息对于早期干预和精准治疗至关重要。
然而,真实世界中的数据显示,尽管多个临床试验已证实NGS能够提高mHSPC患者的生存率,但其应用率仍然较低。在mCRPC中,这一问题同样突出——许多患者未能接受NGS检测,尽管该技术已广泛可用。犹他大学亨茨曼癌症研究所内科肿瘤学部助理教授Umang Swami博士在JAMA杂志子刊JAMA Network Open发表的一项研究显示,美国仅有27%的mCRPC患者接受了NGS检测。在多款PARP抑制剂自2019年起相继获批的情况下,这一比例显得尤为不足。
当前的主要挑战在于提高临床医生和患者对NGS检测重要性的认知,并将最新的临床研究成果推广到更广泛的医疗实践中,而不仅仅局限于ASCO GU、ASCO或ASCR(美国癌症研究学会年会)等学术会议。只有加强医生教育和患者倡导,才能真正改善mCRPC患者的生存质量。
Dr. Neeraj Agarwal: We have seen ongoing discussions about sequencing and next-generation sequencing (NGS) in prostate cancer treatment. The question arises whether we should stop or continue doing NGS testing in our patients, and the answer is clear: we should be offering NGS testing to every single patient with metastatic prostate cancer.
This is an ASCO guideline, and it is supported by nearly every major medical guideline. We cannot compromise on germline testing and NGS testing because the magnitude of benefit with the talazoparib-enzalutamide combination is significantly higher in patients with BRCA1 and BRCA2 mutations, HRR gene alterations, and, to some extent, even in patients without HRR mutations.
Germline testing is also critical because many of these patients have mutations that can have devastating effects on their family members. Knowing these mutations allows us to provide better genetic counseling and targeted interventions.
I am happy to see the final overall survival results from the TALA-PRO2 trial, which we started about eight years ago. It is encouraging to see the benefits in an all-comer population with metastatic CRPC.
Additionally, real-world data indicate that NGS has remained low despite multiple trials since 2013 showing significant survival benefits in metastatic hormone-sensitive prostate cancer. We see the same challenge in mCRPC—patients are not receiving NGS testing, despite its availability. A study led by my colleague, Dr. Umang Swamy, and published in JAMA Network Open, showed that only 27% of patients in the U.S. with metastatic prostate cancer received NGS testing. This is unacceptable, especially since other PARP inhibitors have been approved since 2019.
The challenge remains education and dissemination of level-one evidence beyond ASCO-GU, ASCO, or ASCR meetings. We must take this information to real-world physicians, encourage testing, and improve awareness. Only then will we see real improvement in the overall survival of our patients.

Neeraj Agarwal 教授
犹他大学亨茨曼癌症研究所 (HCI) 医学教授兼捐赠讲席教授
NCCN、ESMO 和 ASCO 泌尿肿瘤指南委员会的小组成员
SWOG GU 委员会的早期治疗负责人
ASCO 泌尿肿瘤顾问小组成员
《ASCO Daily News》主编